LYriCAN+ project
Le LYriCAN + relèvera les principaux défis en l’oncologie et encouragera l’innovation
pour améliorer les soins et le bien être des patients.


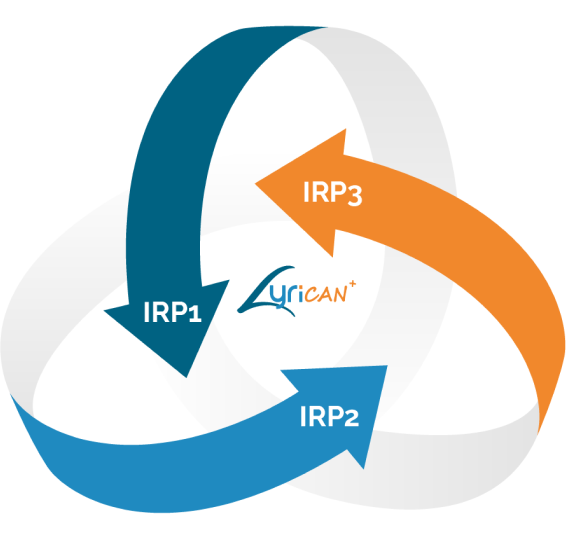
3 programmes
de recherche intégrée
Placés sous la direction d’un binôme clinicien chercheur
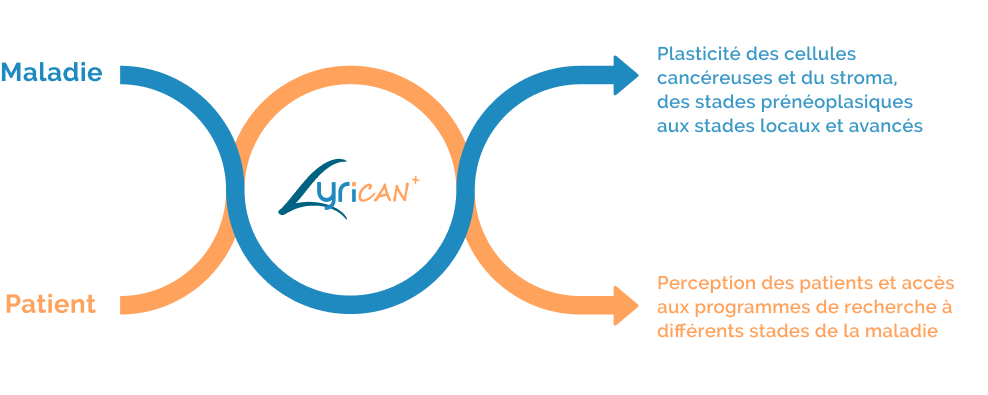
Les retombées
du projet LYriCAN+